Vertex Pharmaceuticals Incorporated Strategy Analysis

Editor-reviewed by Ahmad Zaidi based on analysis by TransforML's proprietary AI
CEO, TransforML Platforms Inc. | Former Partner, McKinsey & Company
Strategy overview for Vertex Pharmaceuticals Incorporated
Vertex Pharmaceuticals Incorporated is a global biotechnology company focused on discovering and developing innovative medicines for serious diseases, particularly in specialty markets. The company has seven approved medicines, including five for cystic fibrosis (CF), one for sickle cell disease (SCD) and transfusion-dependent beta thalassemia (TDT), and one for acute pain. Vertex is committed to extending its leadership in CF and advancing its pipeline across multiple disease areas and modalities.
Key Competitors for Vertex Pharmaceuticals Incorporated
Sionna Therapeutics, Inc.
Developing CFTR modulators, including assets in-licensed from AbbVie Inc.
bluebird bio, Inc.
FDA-approved gene therapy for SCD, LYFGENIA™, and TDT, ZYNTEGLO™
Novartis International AG
Approved small molecule and biologic treatments for SCD and beta thalassemia
Insights from Vertex Pharmaceuticals Incorporated's strategy and competitive advantages
What Stands Out in Vertex Pharmaceuticals Incorporated strategy
Vertex's strategy is uniquely distinguished by its laser-focused, science-first approach of 'depth over breadth,' concentrating on dominating niche specialty markets by targeting the causal biology of diseases. This contrasts sharply with the diversified, broad-portfolio strategies of its larger competitors.
For example, while competitors like AbbVie and Pfizer manage sprawling portfolios across numerous therapeutic areas like immunology, oncology, and internal medicine, Vertex dedicates its identity and resources to achieving near-total market leadership in Cystic Fibrosis (CF) and is replicating this model in other specific, high-unmet-need areas like Sickle Cell Disease (SCD) with CASGEVY.
Furthermore, Vertex's commitment to 'serial innovation'—proactively developing next-generation treatments to replace its own market-leading drugs, such as advancing ALYFTREK while TRIKAFTA is still a blockbuster—is a more aggressive form of internal disruption than competitors who typically focus on offsetting patent cliffs with different assets rather than direct replacements. The pioneering of novel modalities, such as the CRISPR-based gene-editing therapy CASGEVY, is not just a pipeline project but a central pillar of its corporate strategy, making it more foundational to its identity than similar high-science programs within more diversified competitors like Gilead or Johnson & Johnson.
What are the challenges facing Vertex Pharmaceuticals Incorporated to achieve their strategy
Vertex's primary strategic challenge is the significant concentration risk inherent in its focused model; its heavy reliance on the CF franchise creates a vulnerability that diversified competitors do not face. A new, disruptive competitor in CF or a long-term safety issue could be existential, whereas a setback for a single franchise at Pfizer or Johnson & Johnson would be cushioned by their vast portfolios in pharmaceuticals and MedTech.
Secondly, as Vertex moves into curative-intent therapies like CASGEVY, it faces the immense operational challenge of scaling complex manufacturing and commercial models globally. This requires building a new ecosystem of authorized treatment centers and navigating unprecedented reimbursement hurdles for high-cost, one-time treatments, a fundamentally different and more complex task than the established commercial paths for chronic-use drugs sold by competitors like Eli Lilly (Mounjaro) or Amgen (Repatha).
Finally, Vertex must compete for talent and strategic acquisitions against financial goliaths. The aggressive M&A strategy of competitors, such as Amgen's acquisition of Horizon or AbbVie's purchase of Cerevel, demonstrates a level of financial firepower for market entry and pipeline expansion that Vertex cannot currently match, potentially limiting its ability to diversify as quickly as its ambitions require.
What Positions Vertex Pharmaceuticals Incorporated to win
Market Leadership in Cystic Fibrosis
- Vertex has a dominant position in the CF market with five approved medicines, including the recently approved ALYFTREK, treating nearly three-quarters of the approximately 94,000 people with CF in the U.S., Europe, Australia, and Canada.
Successful Commercialization of CASGEVY
- Vertex has successfully launched CASGEVY, a gene-editing therapy for SCD and TDT, securing approvals in multiple geographies and establishing a global manufacturing network and authorized treatment centers.
Approval and Launch of JOURNAVX
- Vertex has expanded its portfolio beyond CF with the approval and launch of JOURNAVX, a non-opioid treatment for acute pain, addressing a significant market with a novel mechanism of action.
Robust and Diversified Pipeline
- Vertex has a broad pipeline of product candidates across multiple disease areas, including AMKD, IgAN, T1D, DM1, and pain, utilizing various modalities such as small molecules, mRNA, and cell and gene therapies.
Strategic Acquisitions and Collaborations
- Vertex has a proven track record of successful acquisitions and collaborations, such as the acquisition of Alpine and collaborations with CRISPR and Moderna, to expand its pipeline and access innovative technologies.
Strong Financial Performance
- Vertex has demonstrated consistent revenue growth and a strong financial profile, enabling continued investment in research and development and business development activities.
Experienced Management Team
- Vertex has a skilled and experienced management team with a proven track record of drug discovery, development, and commercialization.
Strong Intellectual Property Portfolio
- Vertex has a comprehensive intellectual property portfolio protecting its marketed products and pipeline candidates, providing a competitive advantage and market exclusivity.
What's the winning aspiration for Vertex Pharmaceuticals Incorporated strategy
Vertex Pharmaceuticals aims to dramatically advance human health by discovering and developing innovative medicines for people with serious diseases, focusing on specialty markets and validated targets that address causal human biology.
Company Vision Statement:
Company Vision Statement - To discover and develop innovative medicines by combining transformative advances in the understanding of human disease and the science of therapeutics to dramatically advance human health.
Where Vertex Pharmaceuticals Incorporated Plays Strategically
Vertex Pharmaceuticals focuses on specialty markets with significant unmet medical needs, including cystic fibrosis, sickle cell disease, beta thalassemia, acute and neuropathic pain, and APOL1-mediated kidney disease. The company operates globally, with a strong presence in the U.S., Europe, Australia, and Canada, and is expanding into new geographies.
Key Strategic Areas:
How Vertex Pharmaceuticals Incorporated tries to Win Strategically
Vertex Pharmaceuticals wins by combining transformative advances in understanding human disease with therapeutic science, focusing on validated targets, predictive lab assays, and rapid paths to registration. The company leverages a serial innovation approach, internal research, and strategic business development to create first-in-class and best-in-class therapies.
Key Competitive Advantages:
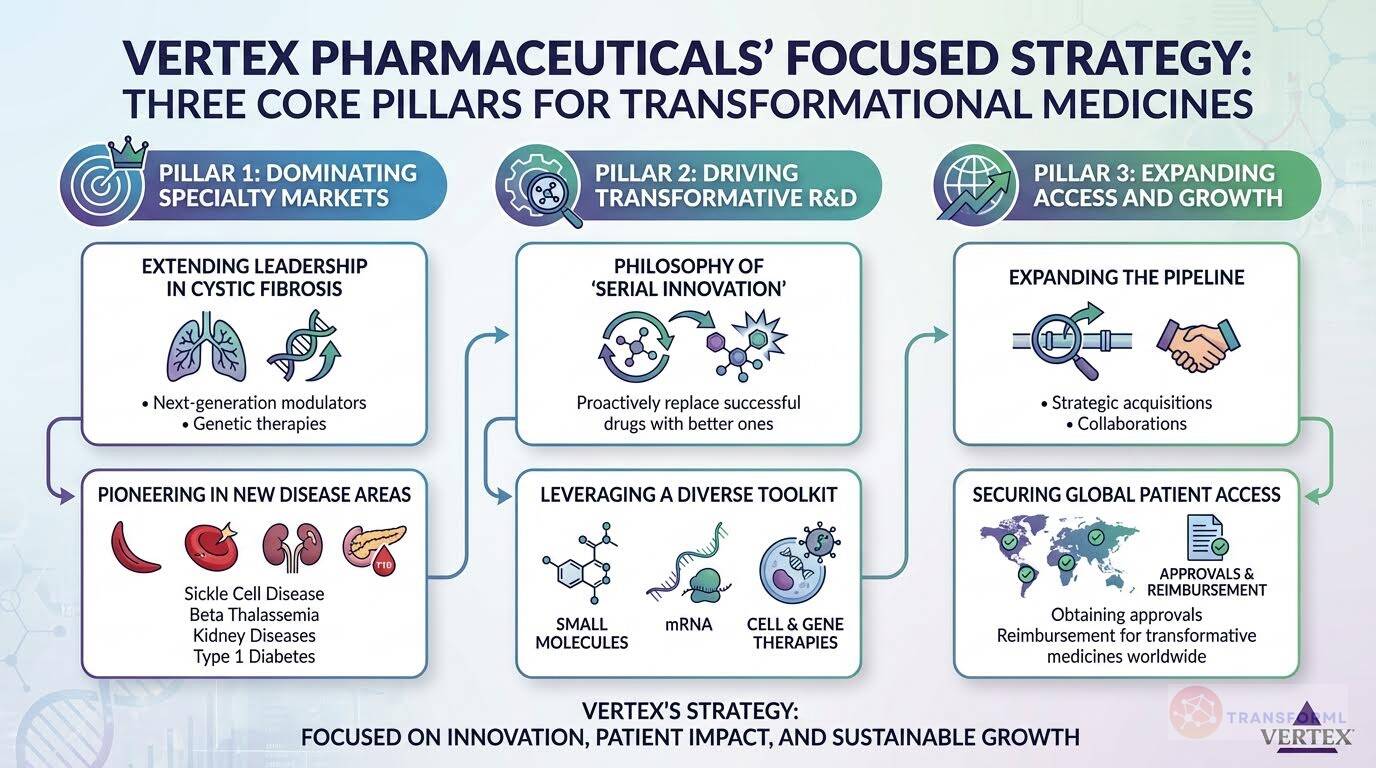
Strategy Cascade for Vertex Pharmaceuticals Incorporated
Below is a strategy cascade for Vertex Pharmaceuticals Incorporated's strategy that has been formed through an outside-in analysis of publicly available data. Scroll down below the graphic to click on the arrows to expand each strategic pillar and see more details:
Related industry articles:
Extend Leadership in Cystic Fibrosis
Continue to develop treatment regimens that will provide benefits to all people with CF, including those who do not make full-length CFTR protein.
Expand ALYFTREK Use to New Patient Segments
Increase the number of patients using ALYFTREK by targeting those currently on other CFTR modulators, those newly eligible, and those who discontinued previous treatments.
Advance Next-Generation CFTR Modulators
Continue identifying and developing additional CFTR modulators with the goal of helping more patients achieve normal levels of CFTR function.
Progress Genetic Therapies for Non-CFTR Protein Makers
Research and develop genetic therapies, such as mRNA, and gene-editing approaches to CF for people who do not make full-length CFTR protein.
Secure Regulatory Approvals for ALYFTREK in Additional Geographies
Obtain regulatory approvals for ALYFTREK in the U.K., the E.U., Canada, Switzerland, Australia and New Zealand.
Expand TRIKAFTA Label to Include Additional Mutations
Pursue regulatory approvals to expand the TRIKAFTA label to include additional non-F508del CFTR mutations.
Eliminate VOCs and Transfusion Dependence in SCD and TDT
Focus on eliminating vaso-occlusive crises (VOCs) and transfusion dependence in patients with SCD and TDT, respectively, through therapies like CASGEVY and investigating small molecules.
Expand CASGEVY Access to Younger Patients
Evaluate CASGEVY as a treatment for children 5 to 11 years of age with SCD or TDT in global Phase 3 clinical trials.
Advance Preclinical Assets for Gentler Conditioning
Progress preclinical assets for gentler conditioning for CASGEVY, which could broaden the eligible patient population.
Investigate Small Molecules for SCD and TDT Treatment
Investigate small molecules for the potential treatment of SCD and TDT.
Secure Additional Regulatory Approvals for CASGEVY
Obtain additional regulatory approvals for CASGEVY in 2025.
Educate Stakeholders on CASGEVY Treatment
Educate physicians, patients and caregivers, payors, and policymakers about the significant disease burden of SCD and TDT and the availability of CASGEVY as a treatment option.
Advance Programs Across Multiple Disease Areas and Modalities
Progress programs across multiple disease areas and modalities, including APOL1-mediated kidney disease, IgA nephropathy, Type 1 Diabetes, Myotonic Dystrophy Type 1, and Autosomal Dominant Polycystic Kidney Disease.
Complete Enrollment in AMKD Interim Analysis Cohort
Complete enrollment in the interim analysis cohort in 2025 and apply for potential accelerated approval in the U.S., assuming a positive interim analysis for inaxaplin in AMKD.
Complete Enrollment in IgAN Interim Analysis Cohort
Complete enrollment in the interim analysis cohort in 2025 and apply for potential accelerated approval in the U.S., assuming a positive interim analysis for povetacicept in IgAN.
Complete Enrollment in T1D Phase 3 Clinical Trial
Complete enrollment and dosing in the Phase 3 portion of the clinical trial in 2025 for zimislecel in T1D.
Advance VX-407 into Phase 2 for ADPKD
Advance VX-407 into a Phase 2 proof-of-concept clinical trial in people with ADPKD in 2025.
Innovate Pain Clinical Trial Design
Innovate in pain clinical trial design to better control the placebo effect, and succeed in pivotal development with suzetrigine.
Secure Broad Access for Approved Therapies
Ensure broad access and reimbursement for approved therapies like ALYFTREK, CASGEVY, and JOURNAVX in eligible patients across various geographies.
Engage Payors for JOURNAVX Reimbursement
Engage with key health care professionals, formulary decision makers and payers to establish the conditions for rapid patient access and uptake of JOURNAVX.
Secure Broad Stocking Agreements for JOURNAVX
Work to secure broad stocking agreements with national retail pharmacies and regional chains, to ensure access to JOURNAVX for patients across the U.S.
Expand Access to CASGEVY Through CGT Access Model
Expand access to CASGEVY by participating in the CGT Access Model for SCD to benefit Medicaid beneficiaries.
Engage with Payors for CASGEVY Reimbursement
Actively engage with relevant stakeholders to obtain broad access and reimbursement with government and commercial payors for CASGEVY.
Maintain Reimbursement for CF Medicines
Focus significant resources to maintain reimbursement and obtain expanded reimbursement for our CF medicines and pipeline therapies in ex-U.S. markets.
Invest in Research and Development
Invest in research and development to discover and develop transformative medicines for people with serious diseases, with a focus on specialty markets.
Advance Multiple Candidates into Clinical Trials
Advance multiple candidates into clinical trials and pursue multiple modalities with the goal of bringing first-in-class and/or best-in-class therapies to patients.
Expand Cell and Genetic Therapy Capabilities
Continue to make significant internal investments in cell and genetic therapies, including the development of a Boston-based campus for research and cGMP clinical manufacturing capabilities.
Pursue Serial Innovation
Pursue multiple modalities with the goal of bringing first-in-class and/or best-in-class therapies to patients.
Develop Follow-on Programs
Develop follow-on programs in our existing disease areas in accord with our serial innovation approach.
Expand Through Strategic Transactions and Collaborations
Expand the pipeline and business through strategic transactions, acquisitions, and collaborations with other entities.
Identify and Evaluate Potential Acquisitions
Continue to identify and evaluate potential acquisitions, licenses and collaborations that may be similar or different from the transactions that we have engaged in previously.
Expand Pipeline Through Acquisitions
Continue to identify and make acquisitions to expand and advance our pipeline and business.
Collaborate with Biopharmaceutical and Technology Companies
Collaborate with biopharmaceutical and technology companies, leading academic research institutions, government laboratories, foundations and other organizations to advance research in our disease areas of interest.
License Technologies Aligned with R&D Strategy
Seek to license technologies, products, product candidates, and businesses that are aligned with our corporate and research and development strategies and complement and advance our ongoing research and development efforts.
Maintain a Strong Financial Profile
Continue to maintain a strong financial profile while investing in serial innovation, launching new products, advancing the pipeline, and expanding geographically.
Manage Global Supply Chain
Continue to build and maintain our supply chain and quality assurance resources as we market and sell our approved products and advance our product candidates through clinical development toward commercialization.
Maintain Reimbursement for Approved Therapies
Continue to focus significant resources to maintain reimbursement and obtain expanded reimbursement for our CF medicines and pipeline therapies in ex-U.S. markets.
Comply with Government Regulations
Actively identify, prevent, and mitigate healthcare fraud and abuse risk through, among other things, the implementation of compliance policies and systems and through the promotion of a culture of compliance.
Manage Financial Risks
Actively manage financial risks, including foreign exchange rate fluctuations, through a foreign currency risk management program.
Read more about industry strategies
Source and Disclaimer: This analysis is based on analysis of Annual reports for 2024. For informational purposes only (not investment, legal, or professional advice). Provided 'as is' without warranties. Trademarks and company names belong to their respective owners.